This research employed an financial mannequin to match the cost-utility of including SGLT2i to SoCT in contrast with SoCT alone for T2D sufferers with CKD utilizing societal perspective to conform to Thai well being know-how evaluation (HTA) guideline suggestions16.
Cohort inhabitants
This research included sufferers recognized with CKD in keeping with the KDIGO 2012 pointers17. The analysis required two irregular outcomes of a GFR of lower than 60 ml/min/1.73 m2 and/or albuminuria. Sufferers with a earlier CKD analysis have been additionally included. Sufferers entered the mannequin at aged 60 years and above for all SGLT2i, based mostly on a earlier research in Thailand that indicated that is the common age of T2D sufferers with CKD18. Moreover, the preliminary distribution amongst CKD phases 3–5 was set uniformly throughout all three SGLT2i, guaranteeing that every therapy group started with comparable baseline CKD stage proportions.
Intervention and comparator
Primarily based on their demonstrated efficacy in RCTs9,10,11, low doses of all accessible SGLT2i in Thailand, together with dapagliflozin 10 mg, empagliflozin 10 mg and canagliflozin 100 mg, have been evaluated. These have been in contrast with SoCT alone, which embrace way of life modification, optimum management of blood strain and lipid in addition to optimum dose of angiotensin-converting enzyme inhibitor (ACEI) or an angiotensin receptor blocker (ARB).
Mannequin construction
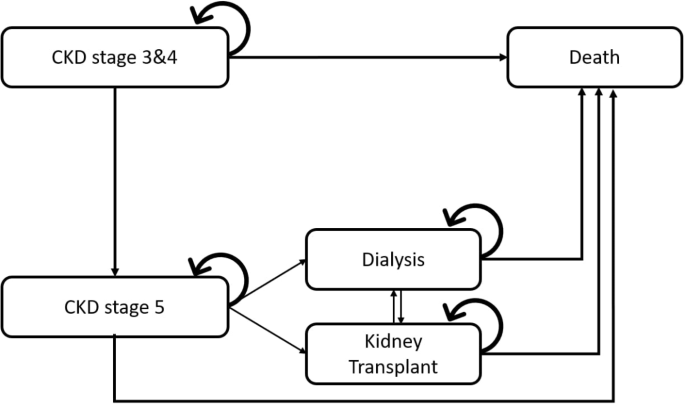
The life time Markov mannequin was constructed to simulate the development of CKD based mostly on a earlier research9,10,11 and medical follow guideline suggestions4,19. The mannequin contains 5 states: CKD phases 3, 4 (sufferers with a GFR of 15–59 ml/min/1.73 m2), CKD stage 5 (sufferers with a GFR of lower than 15 ml/min/1.73 m2), kidney transplantation (sufferers present process RRT through KT), dialysis (sufferers present process RRT through peritoneal dialysis or hemodialysis), and demise, as depicted in Fig. 1. The arrows symbolize the annual possibilities of transitioning between these mutually unique well being states, based mostly on the cycle size. Sufferers can enter the mannequin in both CKD stage 3, 4 or CKD stage 5. Sufferers with CKD stage 3,4 might stay in the identical stage or progress to CKD stage 5. At CKD stage 5, sufferers might progress to obtain RRT through KT or dialysis. Sufferers within the KT state who don’t reply to the therapy might revert to dialysis, and sufferers within the dialysis state can even transition to the KT state. Sufferers can progress to demise from any well being state within the mannequin.
A lifetime Markov mannequin representing the development of continual kidney illness (CKD) in sufferers with sort 2 diabetes. CKD phases 3, 4 are outlined as a GFR of 15–59 ml/min/1.73 m2, and CKD stage 5 is outlined as a GFR of lower than 15 ml/min/1.73 m2. Sufferers enter the mannequin at both CKD stage 3, 4 or CKD stage 5, with a cycle size of 1 12 months.
Mannequin enter parameters
Transitional likelihood
Likelihood of development from CKD 3,4 to CKD 5
A WebPlotDigitizer (model 4.6, 2020) was used to extract knowledge of the development of CKD 3, 4 to CKD stage 5 in Thai inhabitants from a earlier research18 Primarily based on the outcome, this may be represented as an equation exhibiting the connection between the likelihood of creating kidney failure (CKD stage 5) and the variety of years because the onset of CKD as follows: Likelihood of creating CKD stage 5 = (− 7 × 10− 5 × years3) + (0.0013 × years2) + (0.0171 × years) + 0.0046. “12 months” refers back to the length a affected person has lived with CKD phases 3–4 earlier than progressing to stage 5, see Supplementary Fig. 1.
The likelihood of receiving renal substitute remedy, together with KT and dialysis, in sufferers with CKD stage 5
A WebPlotDigitizer (model 4.6, 2020) was utilized to extract knowledge from Thammatacharee et al.20, which presents the charges of dialysis and KT within the Thai inhabitants, categorized by age on the time of remedy. The result’s proven in Desk 1.
Likelihood of demise from CKD stage 3, 4 and CKD stage 5
Mortality knowledge for T2D with CKD sufferers who weren’t handled with SGLT2i, with a GFR of ≤59 ml/min/1.73 m2 and who had not but undergone RRT, was obtained from a research by Vejakama et al.18. This research collected knowledge from T2D Sufferers with CKD in Ubon Ratchathani province, Thailand. Among the many 13,581 sufferers with a GFR between 15 and 59 ml/min/1.73 m2, 900 died inside the median follow-up interval of 4.5 years, leading to a 1-year mortality likelihood of 0.064. For the 384 sufferers with a GFR < 15 ml/min/1.73 m2, 134 died inside the median follow-up interval of 4.5 years, leading to a one-year mortality likelihood of 0.295, Desk 1.
Likelihood of demise from dialysis and kidney transplantation
To handle the shortage of knowledge particular to the Thai inhabitants, we utilized hazard ratios for KT and dialysis mortality beforehand reported in an Asian inhabitants21. The research in reference offered mortality knowledge for people present process dialysis and KT in comparison with the final inhabitants in a Korean context. These findings have been instrumental in calculating the age-specific standardized mortality charges for dialysis and KT in our mannequin, see Desk 1.
Medical efficacy
Since earlier medical trials on the efficacy and security of SGLT2i didn’t deal with T2D sufferers with CKD and didn’t report outcomes in keeping with the well being states in our mannequin, we have been unable to extract efficacy knowledge particular to those well being states. To handle this limitation, we carried out subgroup analyses of related landmark RCTs9,10,22,23 that matched our goal inhabitants (T2D affected person with CKD). Nonetheless, we acknowledged that the smaller variety of individuals in these subgroup analyses might result in underpowered outcomes. To mitigate this concern, we allowed pharmaceutical firms to incorporate all individuals from any landmark RCTs that aligned with our goal inhabitants and supply outcomes related to our mannequin’s well being states. These knowledge have been obtained from AstraZeneca (dapagliflozin 10 mg), Boehringer Ingelheim (empagliflozin 10 mg), and Merck and Janssen (canagliflozin 100 mg). The subgroup evaluation for dapagliflozin primarily drew upon knowledge from DAPA-CKD9, DELCARE-TIMI14, and DAPA-HF trials24; for empagliflozin, from the EMPA-KIDNEY10 and EMPA-REG OUTCOME trials12; and canagliflozin, from the CREDENCE trial11,22,23. The outcomes of those analyses are proven in Supplementary Desk 1 and Desk 1.
Price
Direct medical prices and direct non-medical prices have been included for evaluation in keeping with Thai HTA guideline that recommends to make use of a societal perspective for financial analysis research aiming for coverage advice16. These prices have been adjusted to the 12 months 2023 utilizing the Shopper Worth Index (CPI) for medical care25. Conversion fee of 36.9 Thai Baht per U.S greenback26.
Direct medical value
Direct medical prices embrace remedy prices, laboratory check prices, hospital service prices, and therapy prices. These direct medical prices are derived from three sources together with earlier research in Thailand27,28, unit prices of medical companies from customary value lists29, and the value of SGLT2i, that are referenced from the median costs offered by the Drug Info Heart, Ministry of Public Well being30 (Desk 2).
Direct non-medical value
Direct non-medical prices embrace journey, meals, and lodging bills for sufferers and caregivers associated to therapy. These prices have been obtained from affected person and caregiver interviews at Siriraj hospital between October 1, 2022, and March 31, 2023, and from the usual value checklist for HTA29. The result’s proven in Desk 2.
Utility
The Thai model of the EQ-5D-5 L questionnaire was employed to acquire utility scores (hybrid mannequin) by means of direct interviews with CKD sufferers in varied well being states who visited Siriraj hospital from October 1, 2022, to March 31, 2023. The traits of the included individuals are introduced in Supplementary Desk 2. The imply and customary error (SE) of every well being state is detailed in Desk 2.
We obtained subgroup evaluation outcomes particular to our research inhabitants (T2D with CKD) for medical efficacy and adversarial occasions, together with main hypoglycemia, genital infections, urinary infections, fractures, amputations, and quantity depletion, as reported in medical research9,11,14. Knowledge indicated no distinction in adversarial occasions between teams receiving and never receiving SGLT2i (see Supplementary Desk 3). A current meta-analysis additionally discovered no vital distinction in drug discontinuation charges for SGLT2i (RR 1.03, 95% CI 0.94–1.13)31. Thus, we didn’t embrace SGLT2i negative effects within the evaluation mannequin.
Outcomes
Base case evaluation
The outcomes are introduced because the incremental cost-effectiveness ratio (ICER). This evaluation determines the extra value per quality-adjusted life 12 months (QALY) gained when utilizing an SGLT2i together with SoCT in comparison with SoCT alone. A QALY represents the variety of years lived adjusted for utility. The calculation is as follows: ICER = (whole value of SGLT2i group − whole value of SoCT group)/(QALY of SGLT2i group − QALY of SoCT group). If the ICER is decrease than Thailand’s willingness-to-pay (WTP) threshold of 160,000 Baht or $4,336 per QALY16, it’s thought of cost-effective. Primarily based on Thailand’s HTA pointers, a reduction fee of three% per 12 months was utilized to each prices and outcomes16. The proportion of people in every well being state is introduced in Supplementary Desk 4.
Sensitivity evaluation
This research analyzes the uncertainty of all variables utilizing two strategies: one-way sensitivity evaluation and probabilistic sensitivity evaluation.
One-way sensitivity evaluation
This technique varies one variable of curiosity at a time whereas preserving different variables within the mannequin fixed. The vary of variable variation on this research is calculated utilizing the Bayesian interval technique to estimate the 95% confidence interval (95% CI) for every variable, figuring out the decrease sure and higher sure values. This helps determine which variables considerably affect modifications within the ICER. Moreover, the low cost fee was adjusted to 0% and 6% per 12 months, with the outcomes introduced in a twister diagram32,33.
Probabilistic sensitivity evaluation
This technique makes use of Monte Carlo simulation with Microsoft Workplace Excel 2010 (Microsoft Corp., Redmond, WA). The simulation includes randomly choosing values for every variable and repeating the method 1,000 instances, permitting all mannequin variables to fluctuate concurrently in keeping with their pure knowledge distributions: Beta (for knowledge between 0 and 1), Gamma (for knowledge better than 0 to +∞), and Log regular (for knowledge better than 0, round 1.0, or better than 1). The outcomes are introduced as the common value, well being outcomes, and ICER. The findings are displayed utilizing cost-effectiveness acceptability curves, which present the connection between the likelihood that every possibility is cost-effective and totally different willingness-to-pay thresholds per further QALY gained.
Greatest-case evaluation
Since we derived the medical efficacy of every SGLT2i from subgroup analyses of main RCTs, the small variety of individuals would possibly result in inadequate energy to detect the medical advantages of the remedy, particularly its efficacy in lowering dialysis and kidney transplant. Subsequently, a best-case evaluation using medical efficacy knowledge from beforehand revealed research was carried out.
Ethic approval and consent to take part
The research was carried out in accordance with the Declaration of Helsinki34. The research protocol was permitted by the Institutional Evaluate Board of Siriraj hospital, Mahidol College (MU-MOU CoA 628/2022) to make sure adherence to moral requirements all through all phases of the analysis. Knowledgeable consent agreements have been obtained from all individuals.